STUDY DESIGNS
ERASURE1,2
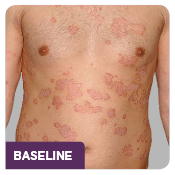
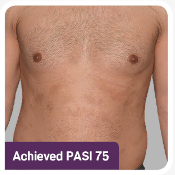
The ERASURE study was a multicenter, randomized, double-blind, placebo-controlled trial of 738 patients.1 Patients were randomized to one of 3 arms: COSENTYX 300 mg* (n=245), COSENTYX 150 mg* (n=245), or placebo† (n=248).1,2 All patients were adults with moderate to severe plaque psoriasis who had a BSA ≥10%, PASI score ≥12, and IGA mod 2011 score ≥3 and were candidates for systemic therapy or phototherapy.2 Patients were followed for up to 52 weeks.1 Coprimary end points were the proportion of subjects who achieved a reduction in PASI score of ≥75% (PASI 75) from baseline to Week 12 and treatment success (clear or almost clear) on the IGA mod 2011 at Week 12.2 Other evaluated outcomes included the proportion of subjects who achieved a reduction in PASI score of ≥90% (PASI 90) from baseline at Week 12, maintenance of efficacy (PASI 75 and IGA mod 2011 clear or almost clear) to Week 52 in patients who were responders at Week 12, and improvements in itching, pain, and scaling at Week 12 based on the Psoriasis Symptom Diary©.2
*Initial dosing for COSENTYX: once weekly for 5 weeks, Weeks 0, 1, 2, 3, and 4; maintenance: once every 4 weeks, Weeks 8 through 48.2
†Placebo nonresponders (who did not achieve PASI 75 at Week 12) were rerandomized 1:1 to COSENTYX 150 mg or COSENTYX 300 mg at Weeks 12, 13, 14, 15, and 16, followed by the same dose every 4 weeks.1,2
CLARITY3,4
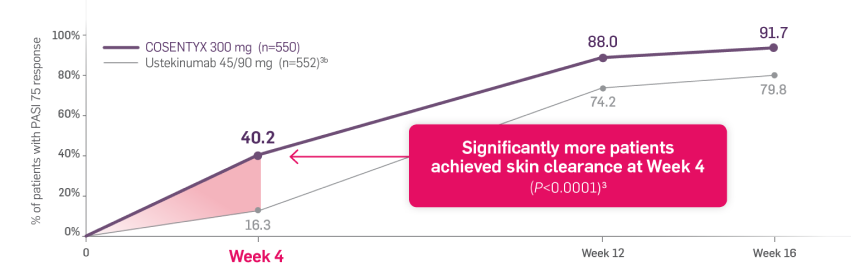
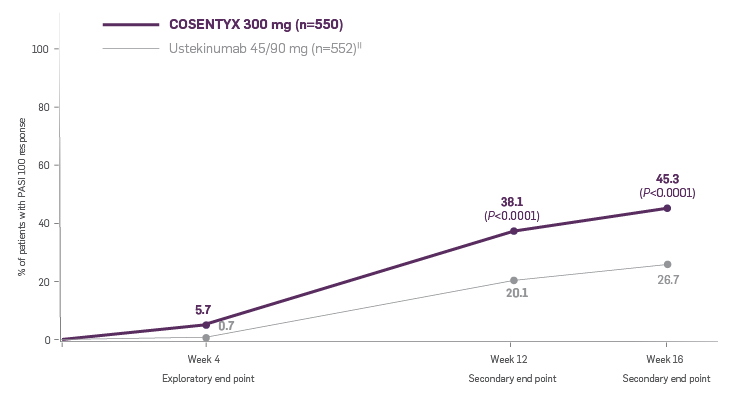
CLARITY is a 52-week, multicenter, randomized, double-blind study of COSENTYX 300 mg‡ (n=550) to demonstrate efficacy as assessed by Psoriasis Area and Severity Index and Investigator’s Global Assessment after 12 weeks of treatment, compared to ustekinumab 45/90 mg§ (n=552), and to assess long-term safety, and efficacy in subjects with moderate to severe plaque psoriasis.3,4
All patients were adults who had a BSA ≥10%, PASI score ≥12, IGA mod 2011 ≥3, and were candidates for systemic therapy or phototherapy.3
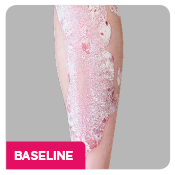
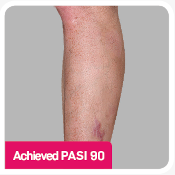
Coprimary end points were the proportion of patients who achieved a reduction in PASI score of ≥90% (PASI 90) and treatment success (clear or almost clear) on the IGA mod 2011 at Week 12.3 Key secondary outcomes included PASI 75 at Week 4, Week 12, and Week 16; PASI 90 at Week 16 and Week 52; PASI 100 at Week 12 and Week 16; and IGA mod 2011 0/1 (clear/almost clear) after Week 16.3
‡Initial dosing: once weekly for 5 weeks, Weeks 0, 1, 2, 3, and 4; maintenance: once every 4 weeks, Weeks 8 through 48.
§Ustekinumab dose was based on body weight at baseline; 45 mg for patients ≤100 kg and 90 mg for patients >100 kg. An active dose was administered at weeks 0, 4, 16, 28, and 40. To maintain blinding, patients received placebo treatments at additional time points.
SCULPTURE Core5
The SCULPTURE Core study was a randomized, double-blind, multicenter trial assessing PASI response and maintenance of response in patients with moderate to severe plaque psoriasis on either an FI regimen (every 4 weeks) or on a retreatment at SoR regimen.5
All patients had a diagnosis of chronic plaque-type psoriasis for at least 6 months prior to randomization; moderate to severe PsO was defined as BSA ≥10%, PASI score ≥12, and IGA mod 2011 score ≥3 and were candidates for systemic therapy or phototherapy.5 During the induction period (Week 0 to 12), patients were randomized to subcutaneous COSENTYX 300 mg¶ (n=484) or 150 mg¶ (n=482); initial dosing weekly at baseline and Weeks 1, 2, 3, 4, and 8.5 At Week 12, patients achieving PASI 75 response were rerandomized to either the FI regimen|| (COSENTYX 300 mg, n=217; COSENTYX 150 mg, n=203) or retreatment at SoR regimen# (COSENTYX 300 mg, n=217; COSENTYX 150 mg, n=206).5
The primary end point was noninferiority of the retreatment at SoR regimen vs the FI regimen for maintaining PASI 75 at Week 52 in patients who were PASI 75 responders at Week 12.5
¶Initial dosing at Weeks 1, 2, 3, 4, and 8.5
||Patients in the FI groups received the same dose they received during the induction period: COSENTYX 150 mg patients continued to receive 150 mg COSENTYX every 4 weeks, and COSENTYX 300 mg patients continued to receive 300 mg COSENTYX every 4 weeks from Week 12 up to and including Week 48.5
#Patients received COSENTYX at Week 12 and then every 4 weeks from the start of relapse (≥20% loss of maximum PASI score improvement vs baseline and a loss of PASI 75 response) until they achieved PASI 75 again.5
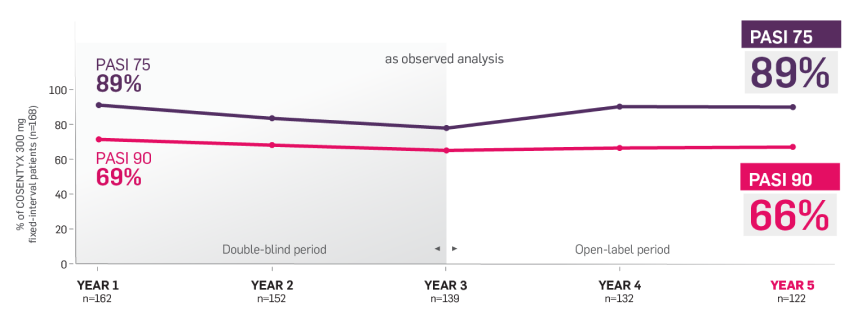
SCULPTURE Extension6
The SCULPTURE Extension study was a multicenter double-blind and open label (from Week 156 through Week 260) trial (N=642).6
Patients who completed 52 weeks of the SCULPTURE Core study were eligible to continue the same COSENTYX dose and regimen in the SCULPTURE Extension study to Week 156 (COSENTYX 300 mg FI [n=168], COSENTYX 300 mg SoR [n=172], COSENTYX 150 mg FI [n=152], COSENTYX 150 mg SoR [n=150]).6 At Week 156, the study was unblinded and, based on investigator judgment, patients could switch dosing regimens (from SoR to FI regimen and from COSENTYX 150 mg to 300 mg).6
Primary objective was to assess long-term safety and tolerability of COSENTYX in patients who completed treatment in the core study. Other objectives included efficacy over time with respect to PASI 50/75/90.6
BSA, body surface area; FI, fixed interval; IGA, Investigator's Global Assessment; PASI, Psoriasis Area and Severity Index; SoR, start of relapse.
References
1. Cosentyx. Prescribing information. Novartis Pharmaceuticals Corp.
2. Data on file. AIN457A2302 Clinical Study Report. Novartis Pharmaceuticals Corp; September 2013.
3. Data on file. CAIN457A2326 Study Protocol. Novartis Pharmaceuticals Corp; April 2016.
4. Data on file. CAIN457A2326 Week 16 Interim Analysis. Novartis Pharmaceuticals Corp; September 2018.
5. Data on file. CAIN457A2304 Clinical Study Report. Novartis Pharmaceuticals Corp; September 2013.
6. Data on file. Clinical Study Report. CAIN457A2304E1. Novartis Pharmaceuticals Corp; February 2018.