COSENTYX has a robust and well-studied safety profile
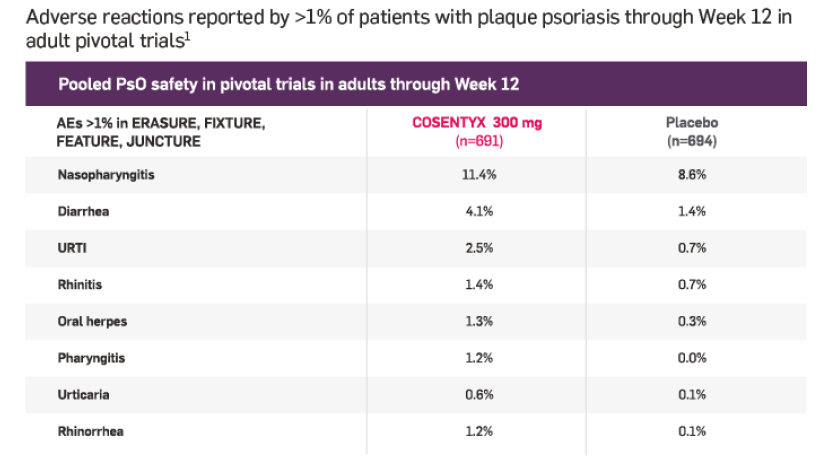
Adult PsO safety profile at Week 121
Infections were reported in 28.7% of patients on COSENTYX (n=1382) vs 18.9% on placebo (n=694)*
Serious infections occurred in 0.14% of patients treated with COSENTYX (n=1382) vs 0.3% of those receiving placebo (n=694)
*Phase III data showed an increasing trend for some types of infection with increasing serum concentration of COSENTYX, including Candida infections, herpes viral infections, staphylococcal skin infections, and infections requiring treatment.1
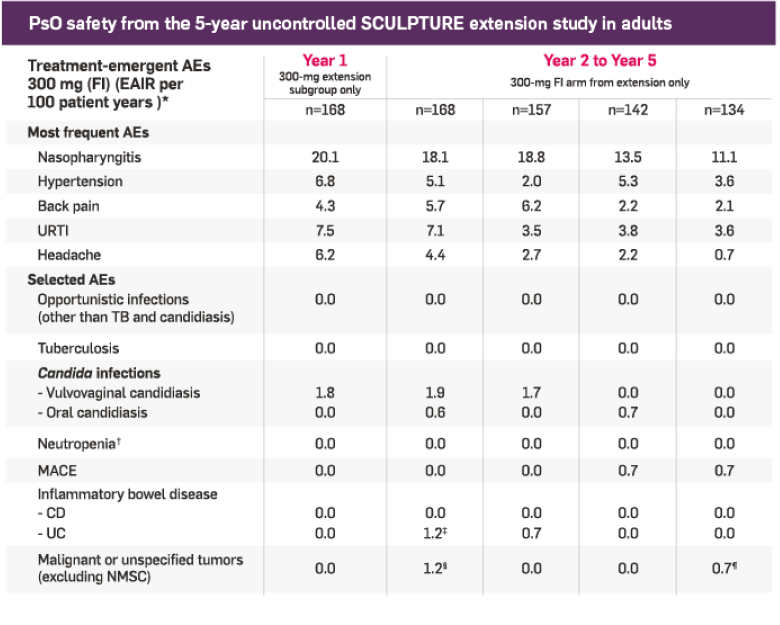
In adults with PsO at Week 12 and at 5 years1,2
In adults with PsA at Week 16 and at 5 years1,3
The safety profile observed in adults with PsA treated with COSENTYX is consistent with the safety profile observed in PsO.1
A robust safety profile1
No new cases of CD and 3 cases of exacerbation (in which 1 patient received COSENTYX); 2 new cases of ulcerative colitis and 2 cases of exacerbation; no cases of IBD in patients with placebo
29% of patients experienced infections (n=1382) vs 19% with placebo (n=694)
0.1% of patients reported serious infections (n=1382) vs 0.3% with placebo (n=694)
If a serious infection develops, discontinue COSENTYX until the infection resolves
A well-studied safety profile1
Safety data up to Year 1 based on a post hoc analysis of the SCULPTURE core study in patients treated with 300 mg who achieved PASI 75 at Week 12, who were rerandomized to 300 mg FI arm, and reconsented at Week 52 to enter the SCULPTURE extension study. Year 2 to Year 5 safety data are presented in this same patient subpopulation; thus, not all patients who started with 300 mg in the SCULPTURE core study were included.
†Patient exposure is calculated as a sum of individual subject durations in days divided by 365 for each interval. A patient with multiple occurrences of the same AE in a 1-year interval was counted only once, while a patient with multiple occurrences of the same AE in different-year intervals was counted for each year.
‡Includes neutropenia adverse events reported by investigators in the SCULPTURE study; does not include lab reports of neutropenia.2
§Of the 2 cases of ulcerative colitis in Year 2, 1 case was an exacerbation of previously existing UC.
¶1 case of cholangiocarcinoma, 1 case of invasive ductal breast carcinoma.
#1 case of breast cancer.
Low immunogenicity
Neutralizing antibodies developed in less than 0.5% of patients at 1 year and were not associated with loss of efficacy1**
**The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease.1
Incidence of inflammatory bowel disease in PsO patients treated with COSENTYX up to 52 weeks1
Of the 3430 patients treated with COSENTYX, 3 experienced Crohn's disease exacerbation, 0 experienced new onset of Crohn's disease, 2 experienced ulcerative colitis exacerbation, and 2 experienced new onset of ulcerative colitis. There were no cases of inflammatory bowel disease in placebo patients (n=793). One case of exacerbation of Crohn's disease was reported from long-term, noncontrolled portions of ongoing clinical trials in PsO.1