For children with moderate to severe plaque psoriasis

Provide skin clearance with COSENTYX® (secukinumab)1
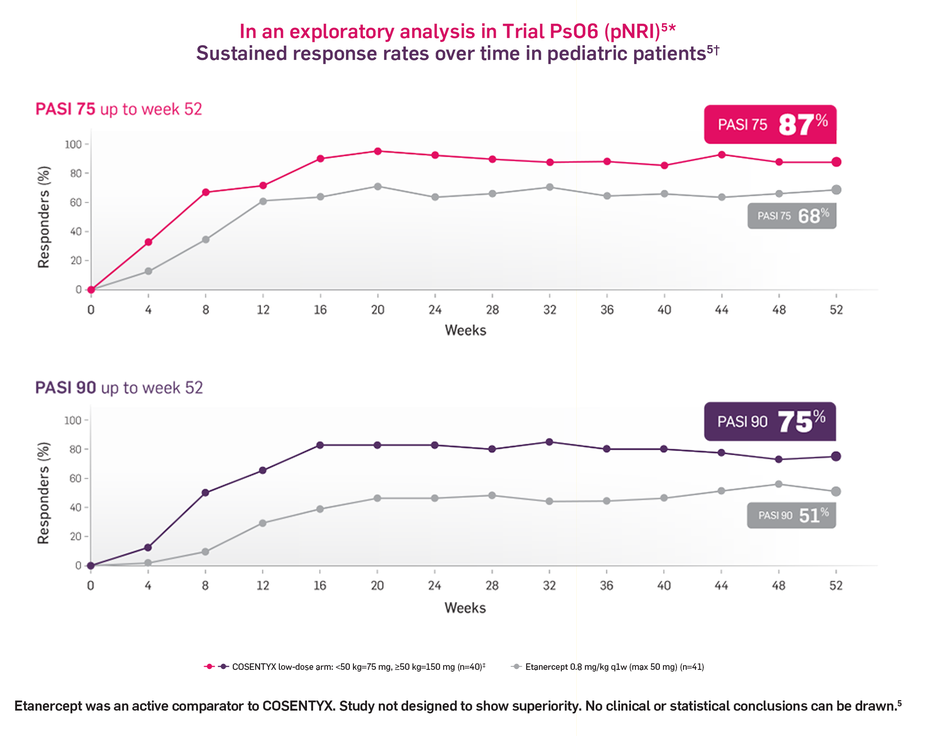
Pivotal Trial PsO6 in pediatric patients with severe PsO
Randomized, double-blind, PBO- and active-controlled trial to demonstrate efficacy and assess safety and tolerability of COSENTYX vs PBO and etanercept (in a single-blinded arm) in subjects from 6 to <18 years of age with severe chronic PsO (defined by PASI score ≥20, an IGA modified 2011 score of 4, and involving ≥10% of the BSA) who were candidates for systemic therapy. Nonresponder imputation (NRI) is used to handle missing values.1
Dosing of COSENTYX1: Patients <50 kg received 75 mg; patients ≥50 kg received 150 mg; administered by subcutaneous injection at Weeks 0, 1, 2, 3, and 4, followed by dosing every 4 weeks.
In exploratory analyses of Trial PsO6
Results observed for COSENTYX low-dose arm†
In Trial PsO6, patients were randomized into two COSENTYX arms:
Low-dose arm: 75 mg for body weight (BW) <50 kg or 150 mg for
≥50 kg.High-dose arm: 75 mg for BW <25 kg, 150 mg for BW ≥25 kg and
<50 kg (2 times the recommended dose), or 300 mg for BW ≥50 kg (2 times the recommended dose).
†COSENTYX low-dose arm: <50 kg=75 mg, ≥150 mg. Data presented here do not account for 3 subjects from the COSENTYX high-dose arm weighing <25 kg who received COSENTYX 75 mg, based on the recommended dosing for pediatric patients <50 kg.
‡In this study, an IRT error led to additional dosing and higher dosing of some patients (LD: n=16; HD: n=20) at Weeks 13, 14, and 15, after the primary end point (Week 12) assessment. This value represents the population not affected by the dosing error.
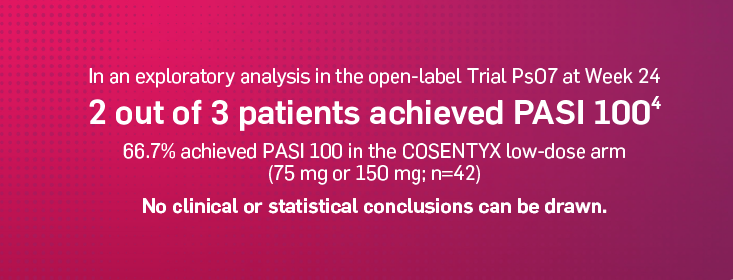
Trial PsO7 in pediatric patients with moderate to severe PsO
Randomized, open-label trial to assess the efficacy, long-term safety and tolerability of COSENTYX compared to historical PBO in subjects from 6 to <18 years of age with moderate to severe chronic PsO (defined by a PASI score ≥12, IGA mod 2011 score of ≥3, and BSA involvement of ≥10% at randomization).4
Patients were randomized into two COSENTYX arms in the same manner as Trial PsO6.
Coprimary end point: low-dose arm (75 mg or 150 mg) at Week 12 (n=42): 93% of patients achieved PASI 75 and 79% achieved IGA 0/1; the estimated probability of a positive treatment effect for COSENTYX vs historical placebo was 100%.4
*PBO-PASI 75 nonresponders at Week 12 were switched to either the COSENTYX low-dose or COSENTYX high-dose treatment group in the maintenance period according to the pre-assignment at their baseline randomization visit. PBO subjects who were PASI 75 responders at Week 12 had to terminate the study.6
†In this study, an IRT error led to additional dosing and higher dosing of some patients (LD: n=16; HD: n=20) at Weeks 13, 14, and 15, after the primary end point (Week 12) assessment. The number of patients in the affected and not-affected patients groups have been deemed to be too low for any differences to be considered as clinically relevant.
‡Data presented here do not account for 3 subjects from the COSENTYX high-dose arm weighing <25 kg who received COSENTYX 75 mg, based on the recommended dosing for pediatric patients <50 kg.
LD, loading dose; PASI, Psoriasis Area and Severity Index; PBO, placebo; pNRI, pure nonresponder imputation; q1w, once per week.
STUDY DESIGNS
PsO61,2
Trial PsO6 is a 52-Week (total 236 weeks) randomized multicenter, double-blind, placebo- and active-controlled phase 3 study in pediatric patients with severe chronic plaque PsO.1
Patients were aged 6 to <18 years1
Severe psoriasis was defined as: PASI score of ≥20, and; IGA score of 4, and; total BSA affected of ≥10%, and; patient being regarded by the investigator to be a candidate for systemic therapy1
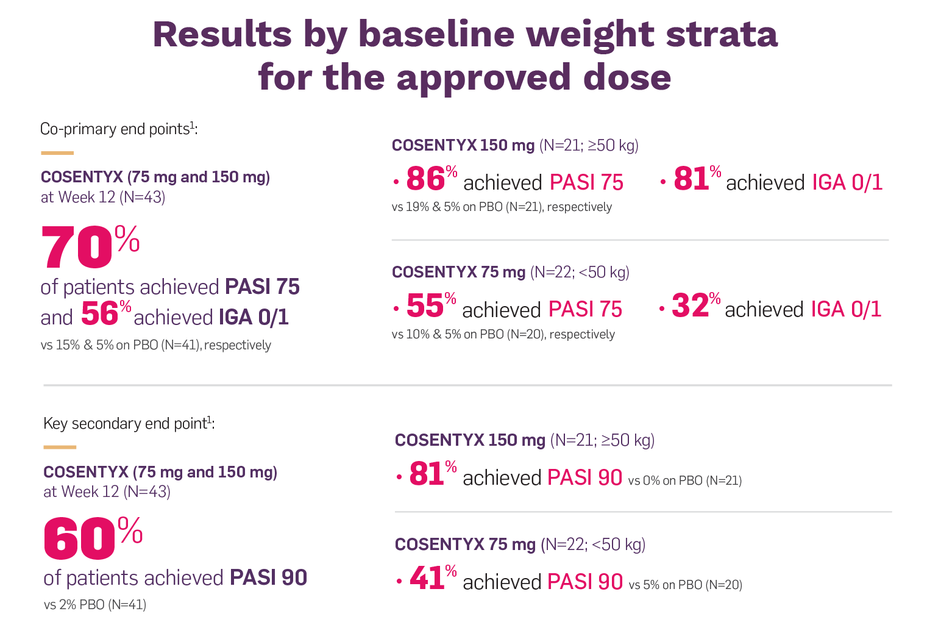
Coprimary end points were the proportion of patients who achieved a reduction in PASI score of ≥75% (PASI 75) from baseline to Week 12 and the proportion of patients who achieved an IGA mod 2011 score of 0 "clear" or 1 "almost clear" with at least a 2 point improvement from baseline to Week 121,2
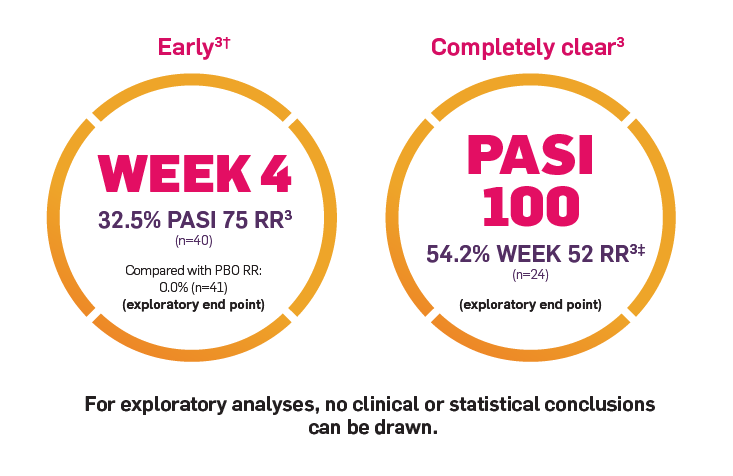
The key secondary end point was the proportion of patients who achieved PASI 90 from baseline to Week 12. Other outcomes evaluated included PASI 100 reponse rates from baseline to Week 12 and through Week 521
PsO73
Trial PsO7 is a 208-week randomized, open label, multicenter study in pediatric patients with moderate to severe chronic plaque PsO.3
Patients were aged 6 to <18 years3
Moderate to severe psoriasis was defined as: PASI score of ≥12 or greater, and; IGA score of ≥3, and; total BSA affected of 10% or greater, and; patient being regarded by the investigator to be a candidate for systemic therapy3
Coprimary end points were the proportion of patients who achieved a reduction in PASI score of ≥75% (PASI 75) from baseline to Week 12 and the proportion of patients who achieved an IGA mod 2011 score of 0 "clear" or 1 "almost clear" with at least a 2 point improvement from baseline to Week 123
The key secondary end point was achievement of PASI 90 reponse rate at Week 12. Other evaluated outcomes included PASI 100 reponse rates over time through Week 243
BSA, body surface area; IGA, Investigator's Global Assessment; PASI, Psoriasis Area and Severity Index; PsO, plaque psoriasis.
References
1. Cosentyx. Prescribing information. Novartis Pharmaceuticals Corp.
2. Bissonnette R et al. Secukinumab demonstrates high sustained efficacy and a variable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE Extension Study). J Eur Acad Dermatol Venereol. 2018; 32(9):1507-1514.
3. Data on file. CAIN457A2304E1 Clinical Study Report. Novartis Pharmaceuticals Corp; February 2018.